Introduction
Vacuoles, E1 enzyme, X-linked, Autoinflammatory, Somatic (VEXAS) syndrome is a newly identified monogenic disorder defined by somatic UBA1 mutations in hematopoietic progenitor cells, resulting in bone marrow failure (BMF) and systemic inflammation. This syndrome is frequently associated with myelodysplastic syndrome (MDS) or plasma cell dyscrasia. Although high-dose corticosteroids alleviate symptoms and cytopenia, allogeneic hematopoietic stem cell transplantation (HSCT) has been suggested as a potentially curative treatment. This study evaluated the safety and efficacy of unrelated HSCT for treating VEXAS syndrome and its prevalence in patients with hematologic diseases.
Methods
We conducted retrospective screening for UBA1 mutations in patients with BMF or plasma cell neoplasms who underwent their first HSCT from unrelated donors in Japan between 1995 and 2020. Pre-HSCT blood DNA samples and clinical data were collected from the Japanese Data Center for Hematopoietic Cell Transplantation. Multiplex real-time PCR was used to detect the most common pathogenic UBA1 mutations, including p.M41L (c.121A>C), p.M41V (c.121A>G), and p.M41T (c.122T>C), with detection limits of 0.13%, 0.5%, and 1%, respectively. The presence of these mutations was confirmed using Sanger sequencing and droplet digital PCR. In addition, we examined UBA1 mutations in 2 patients previously diagnosed with MDS but suspected of having VEXAS syndrome based on specific clinical features.
Results
Among 4340 patients with either BMF or plasma cell neoplasms who underwent unrelated HSCT, 1771 had available pre-HSCT blood DNA samples. The diagnoses of the 1771 patients included MDS (n = 1264), acquired aplastic anemia (n = 375), inherited BMF syndromes (n = 89), plasma cell neoplasms (n = 23), and other acquired BMF syndromes (n = 20). The median patient age at HSCT was 46 (range, 0-75; interquartile range, 20-59) years old, and the male-to-female ratio was 1.6:1.
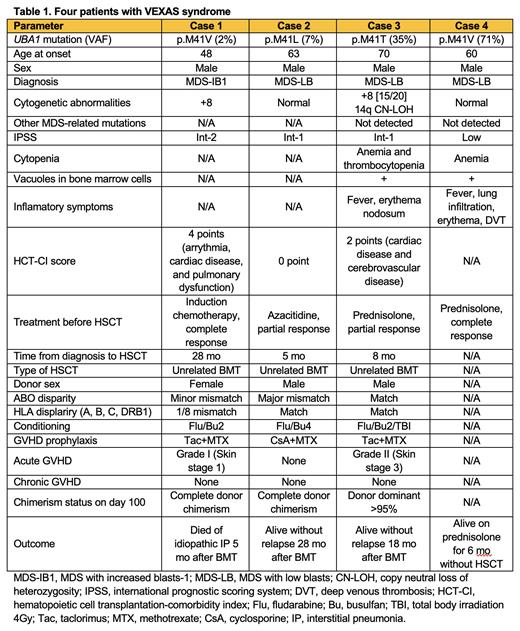
Pathogenic UBA1 mutations were detected in 2 of the 1771 patients (0.11%), both of whom were male MDS patients older than the median age (cases 1 and 2); thus, the prevalence of VEXAS syndrome within this patient subgroup was 0.36%. Both patients received chemotherapy before unrelated donor bone marrow transplantation (BMT), including induction chemotherapy and azacitidine, and achieved complete and partial responses, respectively. In addition, UBA1 mutations were detected in the 2 MDS patients at Kanazawa University Hospital (cases 3 and 4). Case 3 also underwent unrelated BMT after experiencing cytopenia relapse and inflammatory symptoms during corticosteroid reduction. The clinical characteristics and outcomes of the 4 patients with VEXAS syndrome are summarized in Table 1. Variant allele frequencies (VAFs) of UBA1 mutations ranged from 2% to 71%. The 3 patients who underwent unrelated BMT received fludarabine and busulfan-based conditioning regimens and graft-versus-graft disease (GVHD) prophylaxis with tacrolimus + methotrexate or cyclosporine + methotrexate. All 3 patients achieved neutrophil and platelet engraftment. One patient required systemic corticosteroids for acute skin GVHD. One patient died of idiopathic interstitial pneumonia 5 months after BMT. The other 2 patients were alive without relapse, chronic GVHD, or other complications at 18 and 28 months after BMT.
Discussion
Our results support the curative potential of allogeneic HSCT for VEXAS syndrome without excessive toxicity. The occurrence of transplant-related mortality due to idiopathic interstitial pneumonia may be related to the reported poor outcome of the p.M41V mutation following non-transplant therapy. The low prevalence of VEXAS syndrome in the current cohort may be due to the late age of onset and the rare progression to advanced MDS. The low VAFs of UBA1 mutations in the 2 patients who responded to chemotherapy before HSCT may indicate the efficacy of chemotherapy in selectively eradicating UBA1 mutant clones. Our rapid, sensitive, and cost-effective screening method for VEXAS syndrome can help optimize treatment strategies for this rare disease in future studies.
Disclosures
Zaimoku:Nippon Shinyaku: Research Funding. Nannya:Otsuka Pharmaceutical Co., Ltd: Speakers Bureau; Amelieff Corporation: Speakers Bureau; Daiichi Sankyo Company Limited: Research Funding. Hosomichi:GenoDive Pharma Inc.: Consultancy. Doki:Novartis Pharma K.K.: Honoraria; Janssen Pharmaceutical K.K.: Honoraria. Ichinohe:Repertoire Genesis: Research Funding. Atsuta:JCR Pharmaceuticals Co., Ltd.: Consultancy; Meiji Seika Pharma Co, Ltd.: Honoraria; CHUGAI PHARMACEUTICAL CO., LTD.: Speakers Bureau; Otsuka Pharmaceutical Co., Ltd: Speakers Bureau; Novartis Pharma KK: Speakers Bureau. Miyamoto:Kyowa Kirin: Honoraria, Research Funding; AbbVie: Honoraria; Otsuka Pharmaceutical: Honoraria; MSD: Honoraria; Daiichi Sankyo: Honoraria; Novartis: Honoraria; Bristol: Honoraria; Janssen: Honoraria; Astellas: Honoraria; Amgen: Honoraria; Chugai Pharmaceutical: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal